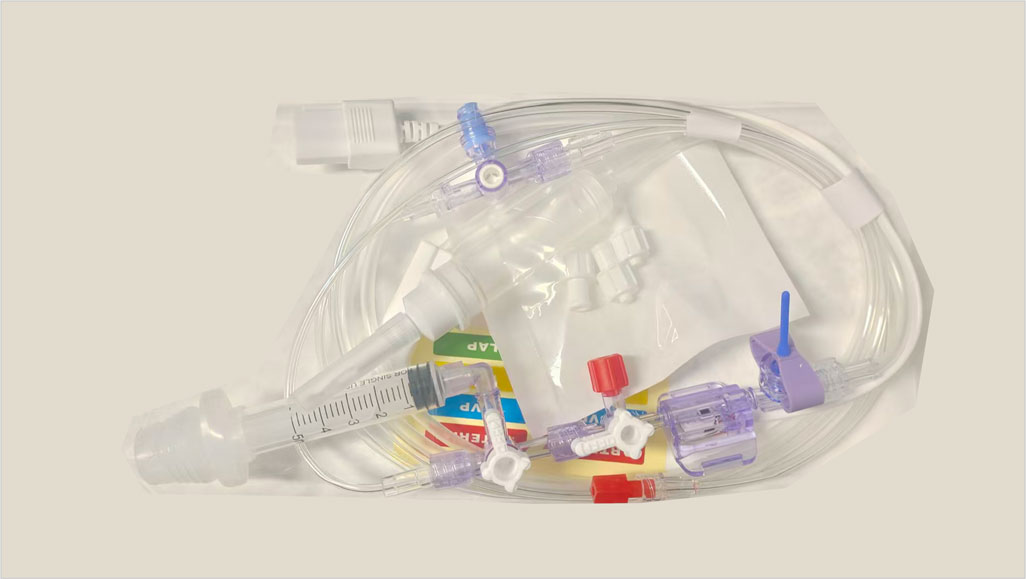
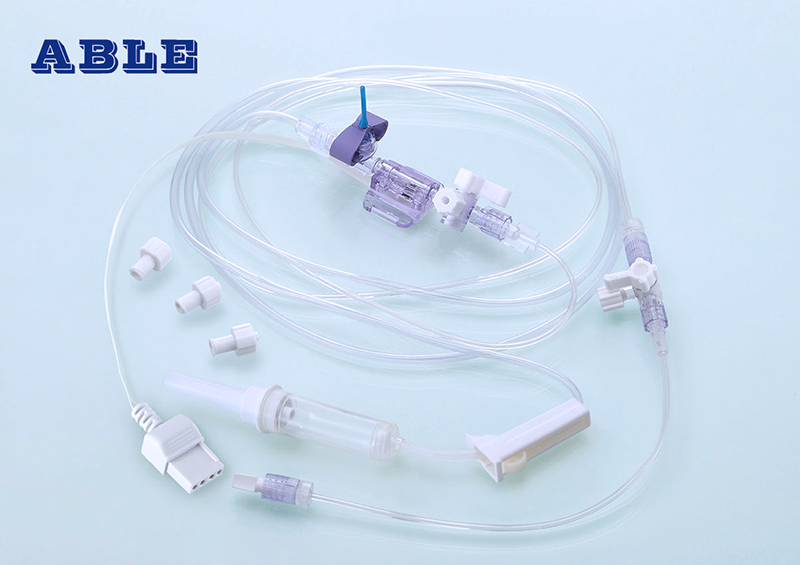
ABLE Disposable Blood Pressure Transducers are mainly composed of a Pressure Transducer, an Infusion Set system, Extension Tube, Protective cap, Flush valve, Stopcocks and a NIBP Cable Connector. The stopcock in the extension tube can act as an open blood sampling site, and some models could include a closed blood sampling site with a limiting syringe.
Intended Use
Specification Model Code Lists
Indications
Product Main Features
Attentions
Contraindication
Validity of Sterilization
Warning
Method of Use
Intended Use: Apply to clinical for traumatic arteriovenous pressure monitoring.
Model
Continued
perfusion flow
Quick perfusion
Color
FT-A, FPT Series
(2~5) ml/h
5 min
Red
FT-V Series
(2~5) ml/h
5 min
Blue
Indications: The device is used on patients requiring arteriovenous pressure monitoringThe device is used on
patients requiring arteriovenous pressure monitoring and could include a closed blood sampling site designed to allow
easy withdrawal of blood samples from an arterial or central venous
pressure monitoring line while maintaining a completely closed system.
Patient Target Group:
1. All kinds of critically patients and surgery patients who are circulatory insufficiency and maybe massive bleeding under the extracorporeal circulation of heart surgery and large blood vessels surgery.
2. Patients with serious hypotension, shock and other blood flow mechanics unstable, and having difficulty by using the indirect method to measure pressure or difficult to measure because of pulse pressure narrow.
Clinical Benefits:
1. Directly measure blood pressure and can reflect the change of blood pressure in each cardiac cycle.
2. Continuous measurements, blood pressure changes can be more timely and accurately grasped, providing reliable basis for the treatment of critically ill patients and improving the success rate of treatment.
Intended Users: Professional health care personnel.
Use Time: For 3 days.
Basic Parameter:
a) The range of environment temperature: 15℃~40℃;
b) The range of relative humidity: 10%~90%;
c) Atmospheric pressure: Unlimited;
d) The range of input pressure: -30mmHg~300mmHg;
e) Transducer excitation voltage: 1V~10V;
f) Excitation voltage frequency: DC- 5 KHz;
g) Sensitivity: 5μV/V/mmHg;
h) Phase Shift: <5°;
i) Light Sensitivity: 1mmHg;
j) Offset Drift: 1mmHg/8h.
Physical Properties:
a) Under the normal temperature and 200Kpa water pressure,
sustain for 15 seconds without the overflowing phenomenon;
b) The connect power of Components ≥15N.
Attentions:
a) The pressure transducer is not applicable to non-insulating monitoring instrument.
b) Do not turn off the sound alarm of pressure transducer.
c) An air filter should be installed in the tube between the pressure transducer and the patient end, when it is applied to measure the pressure of left atrium.
d) Once the monitoring starts, “OFF” on the tap of three-way stopcock must be put on the position of 90 degrees. Do not put it on the position of 45 degrees if you intend to “close”. The position of 45 degrees is not accurate; it may lead to contamination, blood backflow, gas embolism or other dangers.
e) After using normally, we should check the channel every 30 minutes, make sure the pressure of pressure-adding bag is correct, there are not any air bubbles in the channel, the persistent infusion flow rate is normal.
f) Every time after rapid washing, we must watch the dropping speed so as to ensure the persistent infusion flow rate is within the normal range.
g) Ensure the initialized point of the transducer is on the same level of the center of the patient’s heart.
h) We must re-adjust the height of the initialized point of the pressure transducer and initialize it again, once the patient’s body positions changes or the height of the sickbed changes.
i) The disposable blood pressure transducer is the product that can be used one time. It can’t be sterilized again.
j) The electronic connector can’t be dipped in the disinfectant fluid when the repeatedly used connecting cable is disinfected.
k)Do not use the product if accidental dampness.
l) Do not use the product if its performance changes due to aging or environmental changes.
m) Avoid contact with metal objects when using the product.
n) The use of high-frequency electric knife and defibrillator will affect the output waveform of pressure transducer.
o) Do not use if the package is damaged. After use, dispose of the waste products according to the requirements of the hospital or the environmental protection department.
p) The device is an externally communicating device, which may indirect contact with blood path. DEHP has been classified by IARC as a Group 2B Carcinogen-possibly carcinogenic to
humans. There is evidence suggesting that DEHP-induced peroxisome proliferation combined with suppression of hepatocellular apoptosis could be the major molecular mechanism for DEHP-induced hepatocarcinogenicity.
q) The malfunction including product leakage, no output, calibration zeroing failure, may lead to blood lose, or incorrect diagnosis. Please contact to the manufacturer when adverse events happen.
r) Do not use the product when leakage is observed.
s) It is recommended to use the extended cable compatible with monitor to avoid failure to display the reading.
t) When using the pressure sensor, flush the pipeline regularly to prevent clogging.
u) When any serious incident that has occurred in relation to the device, please report to the manufacturer and the competent authority of the Member State in which the user and/or patient is established, the contact information is shown below:
TEL: + 86 757-81207300
FAX: +86 757-81207311
Websit: http://www.baihemedical.com
Email: able@baihemedical.com
Contraindication: It is prohibited to measure the pressure of left atrium in case that the air filter is not installed between the cannula and the persistent infusion valve.
Validity of Sterilization: The validity of sterilization is 3 years.
Warning:
a) Check one time every three months when storing. It is prohibited to use the unqualified product.
b) This pressure transducer is designed to be used within three days. One should determine by oneself when it is used longer than this time limit.
c) All performance is based on the complete product. If the parts are replaced, the use effect cannot be achieved.
Withdrawal of blood sample
1. Turn the tap of the three-way stopcock, which is connecting with limiting syringe, so that “OFF” face to the Transducer.
2. Pull upon the limit syringe until it stops and reaches its 2mL volume capacity.
3. Turn the tap of the three-way stopcock, which is connecting with patient, so that “OFF” face to the Transducer
4. Drawing blood samples from the Blood sampling site.
a) For the Needle free blood sampling site: Disinfect the Blood sampling site firstly and then withdraw the blood sample using a Luer lock syringe to connect with Needle free blood sampling site.
b) For the Normal blood sampling site: Disinfect the Blood sampling site first and then put the blood collecting needle which connect with vacuum tube, into the Normal blood sampling sit to withdraw the blood sample.
5. Flush the Extension tube:
a) Turn the tap of the three-way stopcock, which is connecting with limiting syringe, so that “OFF” face to the Transducer.
b) Push down the limiting syringe until the 0 ml scale, to make sure the flexures lock in place in the fully closed position and all fluid has been reinfused into the transmission tube.
c) Flush the Extension tube:
d) Turn the tap of the three-way stopcock, which is connecting with patient, so that “OFF” face to the Blood sampling site.
6. Check the blood pressure monitor.